While adoptive T cell therapies have led to remarkable clinical responses in certain subsets of B cell leukemia and lymphoma, many challenges remain that are limiting the efficacy in other hematological malignancies. Compromised T cell function and exhaustion due to chronic antigen exposure can impair the persistence of chimeric antigen receptor (CAR)-T and T-cell-receptor (TCR)-T cells. Gene editing strategies have the potential to circumvent some of these challenges and enhance T cell function.
To identify key genes that are involved in regulating T cell dysfunction, we performed a genome-wide CRISPR loss-of-function screen in primary human T cells in conditions that mimic chronic antigen exposure. Primary T cells from two donors were transduced with lentivirus containing a NY-ESO-1 TCR as well as a genome-wide sgRNA library and subsequently CRISPR edited using Cas9-RNP electroporation. These antigen specific TCR-T cells were then co-cultured with the NY-ESO-1+ melanoma cell line A375 at an effector-to-target (E:T) ratio of 1:1 for 48 hours and challenged under the pressure of chronic tumor stimulation for 9 rounds in total. The resulting next-generation sequencing data was then analyzed utilizing the MAGeCK pipeline to identify top scoring hits for validation regarding their role in T cell fitness and persistence.
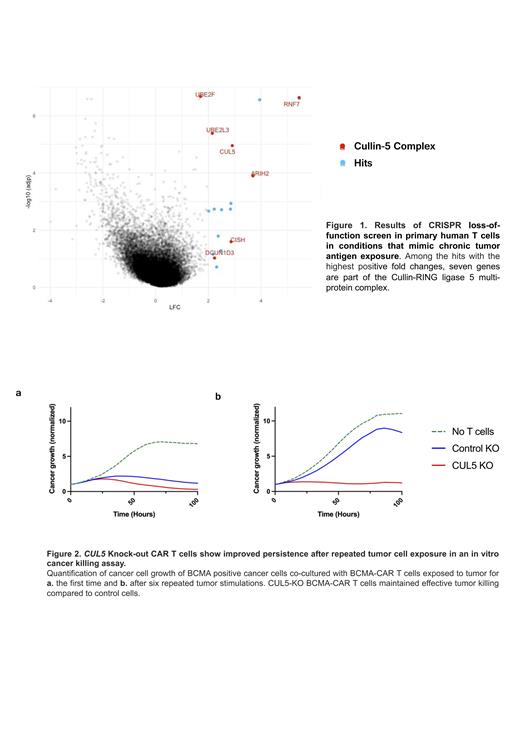
Among the hits with the highest positive fold changes, we found seven genes which are part of the Cullin-RING ligase 5 multi-protein complex (Fig. 1). These hits were then validated in a CAR-T cell context. We first edited primary human T cells by knocking out CUL5 as well as the endogenous TCR. The template for the B Cell Maturation Antigen (BCMA)-specific CAR bb2121 was then delivered to the TRAC locus via CRISPR/Cas9-RNP and adeno-associated virus (AAV). Here, we tested a panel with four CAR modifications, including two different co-stimulatory domains (CD28ζ, 4-1BB), a CAR with mutated immunoreceptor tyrosine-based activation motifs (ITAM) (1XX CAR), and a CAR containing an alternative BCMA-targeting single-chain variable fragment. This panel of CUL5 ablated CAR-T cells were then repeatedly co-cultured with BCMA-expressing tumor cells. After 6 rounds of tumor exposures, CUL5 ablated T cells showed a striking tumor killing advantage compared to control edited cells in a functional tumor killing assay (Fig. 2). Notably, we also observed vastly increased production of the cytotoxic cytokines INF-γand TNF-α in CUL5 ablated cells. Furthermore, the distinct tumor killing advantage of CUL5 KO cells in vitro was reproduced in the context of a CD19-specific CAR, which will be relevant in other clinical settings.
Finally, we evaluated the striking tumor killing capacity of CUL5 ablated BCMA-specific CAR-T cells in vivo. For this, NOD.Cg- Prkdc scid Il2rg tm1Wjl/SzJ mice engrafted with luciferase-expressing BCMA+ multiple myeloma cell line OPM2 were treated with this panel of CUL5 ablated and control CAR-T cells and tumor burden was measured via Bioluminescent Imaging (BLI) over time. Mice treated with CUL5 KO BCMA-specific CAR-T cells showed lower median radiance measured by BLI, except for the 1XX CAR condition where both cohorts completely cleared the disease. In addition to the complete tumor clearance in both the control and CUL5 edited cohorts, we observed that the BLI levels in the 1XX CAR treated animals remained stable even after the mice were subjected to five tumor rechallenges. After 90 days, the mice were sacrificed and CAR+ human T cells were isolated from the bone marrow and spleens and phenotypically assessed. Analysis of these samples revealed a significantly higher frequency of CAR+ cells in the mouse bone marrow in the CUL5 KO group compared to control, while there were equivalent numbers in the spleens, suggesting an antigen-specific expansion advantage for the CUL5 KO CAR-T cells. We then co-cultured the isolated BCMA CAR-T cells from the spleens of these mice with OMP2 tumor cells in different E:T ratios and assessed tumor killing. We found a significant advantage in tumor killing of CUL5 ablated T cells compared to control cells in this ex vivo setting, suggesting an improved functional persistence.
In summary, our findings identify the CUL5 complex as a novel potential therapeutic target to improve T cell persistence and counteract T cell exhaustion in order to improve the efficacy of adaptive cell immunotherapies for the treatment of BCMA+ hematological malignancies.
Disclosures
Eyquem:Resolution Therapeutics: Consultancy; Enterome: Consultancy; Treefrog Therapeutics: Consultancy; Casdin Capital: Consultancy; Takeda Pharmaceutical Company: Research Funding; Indee Labs: Research Funding; Cytovia Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Research Funding; Mnemo Therapeutics: Current holder of stock options in a privately-held company, Other: Compensated co-founder, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal